Changing people's lives
Listening to patients, their families, their care providers, and illness advocacy organizations has directly led to the development of our clinical pipeline.
Evaluating the potential of a novel gene therapy drug candidate which utilizes a trifold effector approach for the treatment of heart failure is the first of its kind in the world.
Listening to patients, their families, their care providers, and illness advocacy organizations has directly led to the development of our clinical pipeline.
Current treatments can improve the quality of life and ensure long-term survival benefits at an efficient cost and with reduced potential side effects.
Cardiovascular Gene Therapy Design Matrix (Balancing Safety and Efficacy)
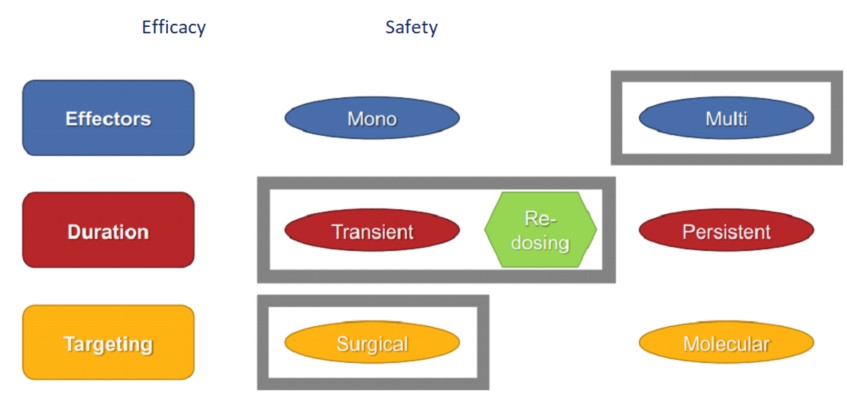
The non-viral administration of multigenic vectors focuses on the pathological cardiac remodeling's underlying molecular pathways.
GENEX-3: A Trifold Effector Plasmid
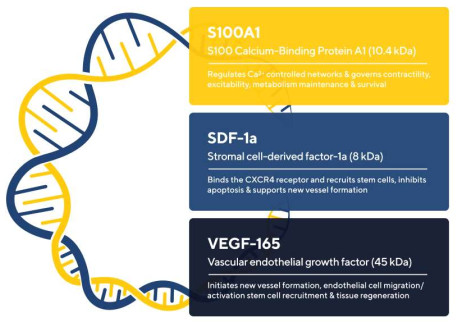
Utility of Multigenic Approach Demonstrated in Human-Diseased Cardiomyocytes
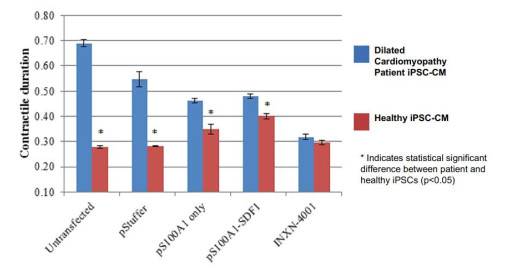
GENEX-3 Transfection Improves Contractile Duration of Patient Cardiomyocytes In-Vitro
In comparison to control cells, DCM iPSC-CM showed a substantial improvement in beat rate, contractile duration, and contraction rate after GENEX-3 transfection, but did not see an increase in cell death.
Favorable Biodistribution and Function of GENEX-3
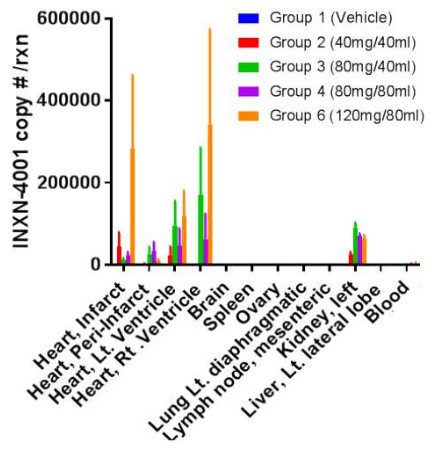
Biodistribution of GENEX-3 Indicates Cardiac-Specific Delivery
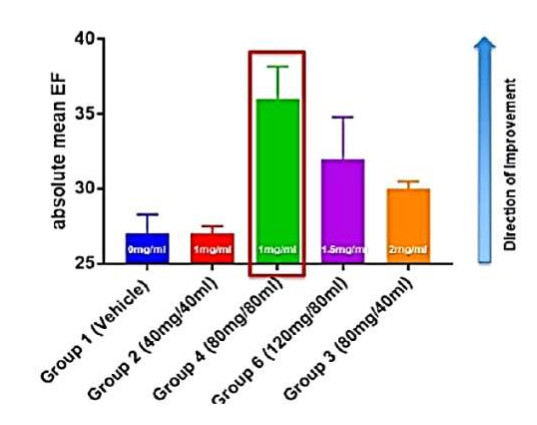
Comparison of Myocardial Functional Parameters Across GENEX-3 Doses Indicates Increase in EF
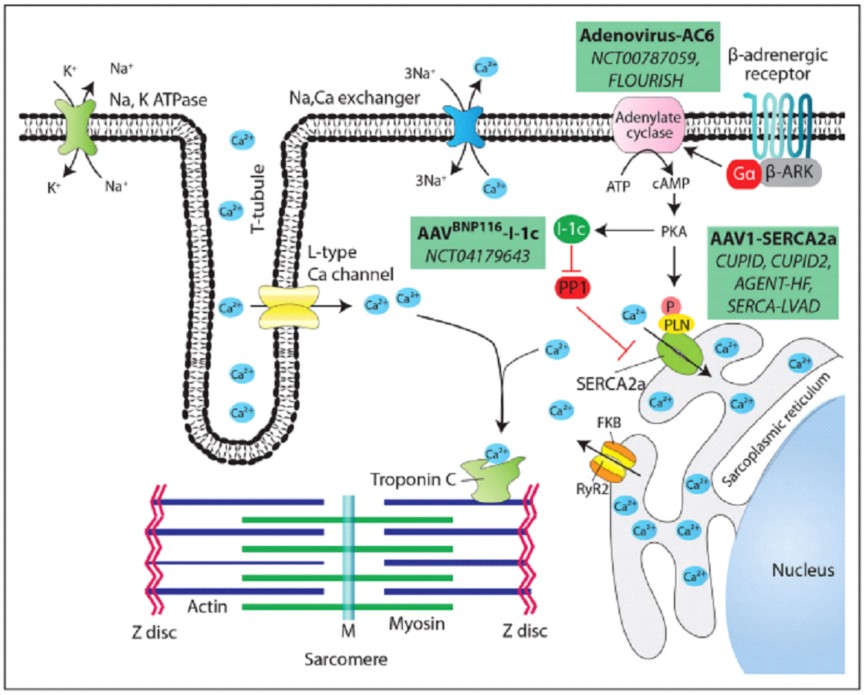
Our current gene therapy strategy, which targets cardiac excitation-contraction coupling via the cardiomyocyte Ca2+ cycle, is shown in the top figure.
The RyR2 ryanodine receptor controls the release of Ca2+ from the sarcoplasmic reticulum stores when the cardiomyocyte plasma membrane depolarizes, causing the membrane's L-type voltage-dependent Ca2+ channels to open and allow a tiny amount of Ca2+ to enter the cytosol. Ca2+ binding to troponin C, which is triggered by a large amount of Ca2+ entering the cytosol, causes biochemical coupling between actin and myosin, which leads to contraction. The FKBP12.6 protein prevents RyR2 from functioning during the relaxation period.
The released Ca2+ is in part re-convoyed into the sarcoplasmic reticulum by the ATPase SERCA2a, and in part eliminated outside the cell by the Na+/ Ca2+ exchanger (NCX). SERCA2a pump activity is regulated by its association with PLN (phospholamban). In its unphosphorylated form, PLB hinders SERCA2a activity; however, phosphorylation of PLB hinders this difficulty. The primary kinase responsible for phosphorylating PLB in cardiomyocytes (promoting pump activation) is PKA (protein kinase A) which is regulated by β-adrenergic stimulation. Specifically, the engagement of β-adrenergic receptors with their ligands activates a heterotrimeric G protein, which activates an AC (adenylate cyclase) located on the cytosolic side of the receptor complex, catalyzing the conversion of ATP to cAMP.
The activation of PKA through a phosphorylation process leads to the modulation of three key components within the cardiac cycle: (1) the L-type Ca2+ channels allowing further calcium entry; (2) RyR2, which promotes the dissociation of the inhibitory protein FKB12.6; and (3) PLB, diminishing its inhibitory effect on SERCA2a. Consequently, these changes amplify the effectiveness of calcium release and reuptake during the cycle. On the other hand, dephosphorylation of PLN leads to the deactivation of SERCA2a, which primarily relies on protein phosphatase-1 (PP1) inhibited by I-1c (inhibitor-1c). The figure illustrates the three main medical approaches to intervene within these pathways in heart failure by transferring the cDNAs coding for SERCA2a, AC6, or I-1c (rectangular green boxes), indicating the vector used and the name of the clinical studies.
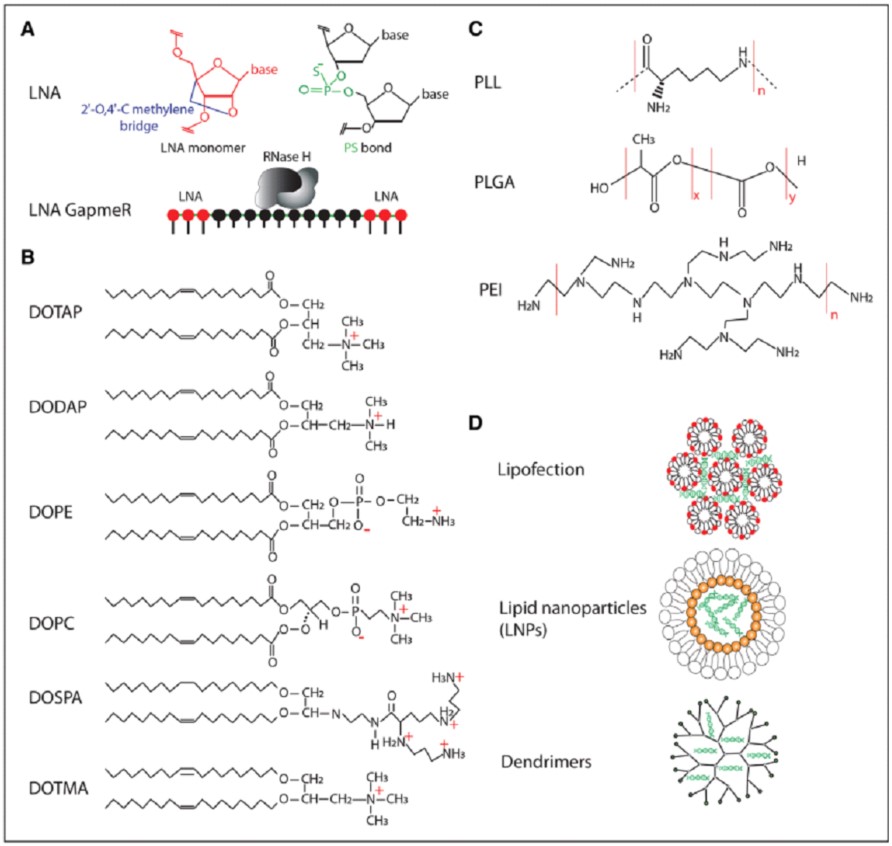
Molecules and methods for the delivery of RNA therapeutics
The upper left part of the panel displays the chemical structure of an LNA nucleotide and a phosphorothioate bond. The lower part of the panel shows the arrangement of an LNA gapmer, wherein the LNA-modified nucleotides are placed at the two ends of the oligonucleotide, allowing RNase H to reach the middle part after the duplex with the target RNA is created.
The principal cationic and neutral lipids utilized for lipofection and formation of lipid nanoparticles can be identified by their chemical structures. Polyplexes are formed from a variety of main polymer materials, including poly(ethylenimine) [PEI], polyL-lysine [PLL], and poly (lactic-co-glycolic acid) [PLGA]. The last part shows a schematic diagram showing the arrangement of DNA and nanoparticle components.
Applications that produce secreted proteins instead of relying heavily on cardiomyocyte transduction, like the release of the SERCA2a cDNA in our most recent studies, are more effective. In cardiac samples from treated patients who underwent transplantation, or had mechanical support device implantation, the amount of AAV1.SERCA2a DNA was determined to be 43 copies per microgram total DNA50 (for comparison, DNA from 1106 mononucleated diploid cells weighs 6 g). Given that SERCA2a functions intracellularly, this was unquestionably a positive outcome. Despite the fact that extravasation from the intact endothelium (paracellular permeability) is more effective for particles with a size of 20 nm diameter, such as AAV virions, vector intracoronary injection, such as in the CUPID trials, is a less invasive approach.
AAV1-SERCA2a's reported a greater amount of empty viral vectors in the CUPID2 trial compared to the initial CUPID study (25% versus 90%, respectively) was another delivery-related efficacity factor.
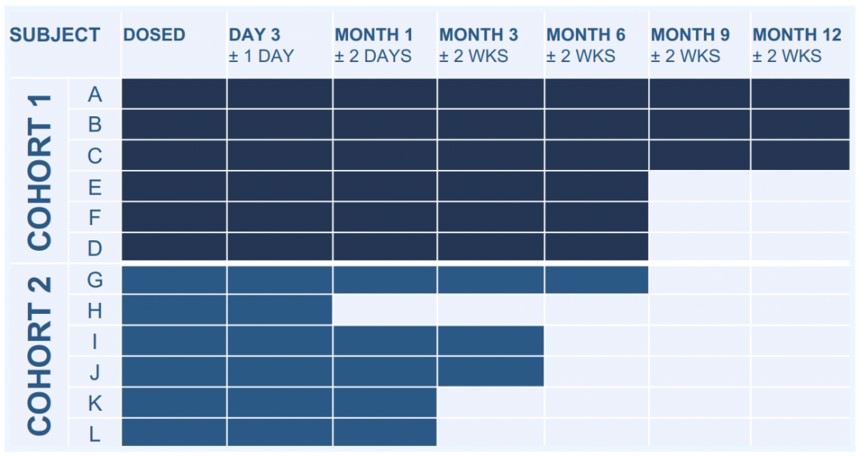
GENEX-3 Phase I Study Design and Status
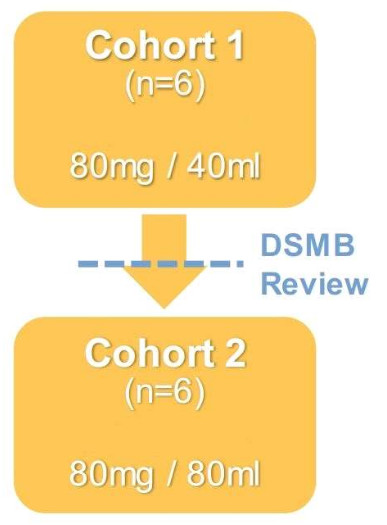
GENEX-3 administered by RCSI in a first-in-human, phase I, open-label, safety study in patients with outpatient LVAD
To assess the safety of administering the same dose of GENEX-3 (80 mg) in two volumes (40 mL and 80 mL) at a rate of 20 mL/min, 12 stable patients with an implanted LVAD for mechanical support of end-stage HF were divided into two cohorts, each with six individuals.
RCSI data were gathered during clinic visits at the following times: pretreatment, day 3, then 1, 3, 6, and 12 months after dosage.
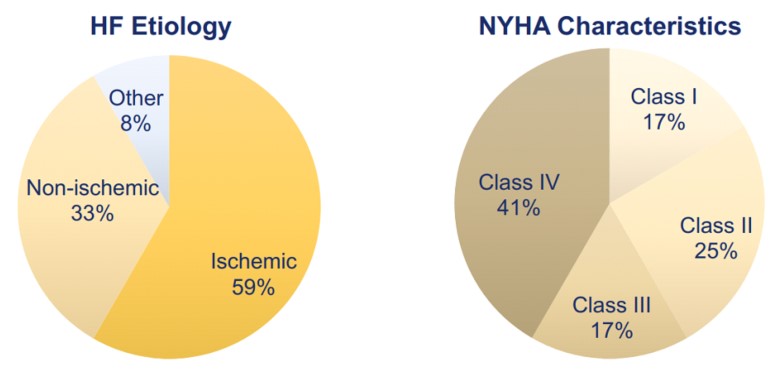
Heart Failure Characteristics at Baseline
Six-Minute Walk Test - Distance Walked ON LVAD
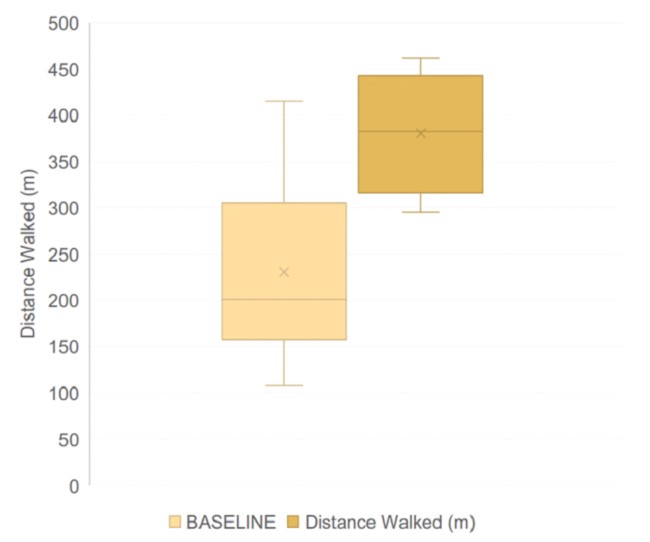
Six-Minute Walk Test Distance
After six months of GENEX-3 treatment, a tendency towards better performance in the six-minute walk test was seen, with an increase in the distance traveled.
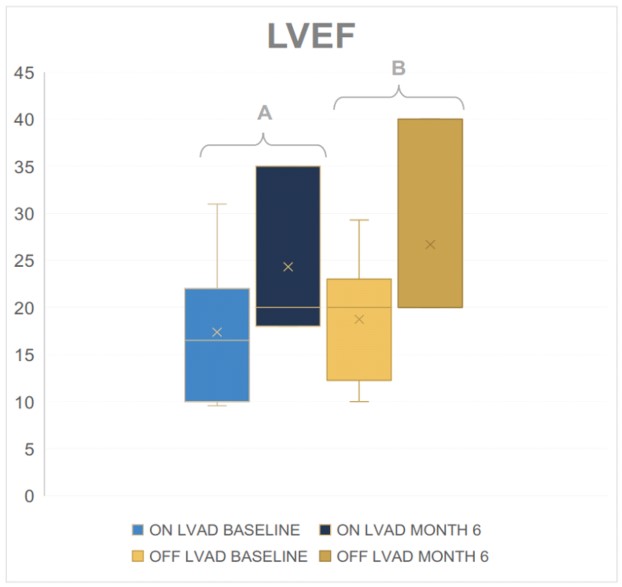
Left Ventricular Ejection Fraction (LVEF)
The GENEX-3 treatment was seen to have a positive impact on the left ventricular ejection fraction (LVEF), which is a measure of the amount of blood pumped out of the left ventricle during each contraction, after 6 months.


